The equilibrium constant expression for this reaction that takes place in water and involve ions can be written as K= [H_3 O^+ ][OH^- ]/([H_2 O] ^2 ). But the concentration of undissociated water, H_2 O is much larger than the concentration of the ions that is essentially remains constant. Therefore, we can include it in the equilibrium constant. The resulting new equilibrium constant can be written: K_W= [H_3 O^+ ][OH^- ].
Water undergoes auto-ionization according to the following equation:
H2O(l) + H2O(l) 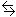 H3O+(aq) + OH-(aq)
H3O+(aq) + OH-(aq)
or
2 H2O(l)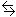 H3O+(aq) + OH-(aq)
H3O+(aq) + OH-(aq)
The equilibirum expression for the above reaction is written below and is treated mathematically like all equilibrium expressions.
At 25oC, the value of Kw has been determined to be 1 x 10-14. This value, because it refers to the auto-ionization of water, has been given a special symbol, Kw, but, it is just a special case of Kc.
If one knows the concentration of either the hydronium ions or of the hydroxide ions in a water solution,. the other ion concentration can be determined.
The Auto-Ionization of Water, Kw
or
2 H2O(l)
Kw = [H3O+][OH-]
If one knows the concentration of either the hydronium ions or of the hydroxide ions in a water solution,. the other ion concentration can be determined.